Mineral Information
 Calcite — CaCO3
Calcite — CaCO3
A mineral consisting largely of calcium carbonate. Next to quartz, it is the most abundant of the earth's minerals. Crystallizing in the hexagonal system, calcite is noted for its wide variety of crystalline forms.
Uses:
- Animal feed
- Antacid - from calcium carbonate
- Building construction
- Chemical industry
- Dough strengthener
- Facing stone for building interiors/exteriors
- Filter in baking powder
- Glass industry
- Manufacturing of paper and the paper industry
- Optical purposes
- Photography
- Statues
- Waste treatment
 Pyrite — FeS2
Pyrite — FeS2
Pyrite is the classic "Fool's Gold." There are other shiny, brassy yellow minerals, but pyrite is by far the most common and the most often mistaken for gold. Whether it is the golden look or something else, pyrite is a favorite among rock collectors. It can have a beautiful luster and interesting crystals. It is so common in the earth's crust that it is found in almost every possible environment; hence it has a vast number of forms and varieties.
Pyrite is a polymorph of marcasite, which means that it has the same chemistry as marcasite, but a different structure and therefore different symmetry and crystal shapes. Pyrite is difficult to distinguish from marcasite when a lack of clear indicators exists.
Uses:
- Pyrite was once used as the main source of sulfur, but is now only a minor source for sulfur and iron. Pyrite from some localities is auriferous, and therefore is used as an ore of gold.
- Pellets of pressed pyrite dust have been used to recover iron, gold, copper, cobalt, nickel, etc.
- Pyrite was polished by Native Americans in early times and used as mirrors. Today, it is used as an ornamental stone, as well as a very popular stone for the amateur collector. It is sometimes used as a gemstone by being faceted and polished for use as a side jewel in a ring, necklace, or bracelet.
 Elbaite Tourmaline — (Na,Ca)(Mg,Li,Al,Fe2+)3 Al6 B3 Si6(OH)4
Elbaite Tourmaline — (Na,Ca)(Mg,Li,Al,Fe2+)3 Al6 B3 Si6(OH)4
Tourmaline is actually a group of several different minerals that have similar crystal structures, but complex and variable chemical formulas. There are eleven distinct mineral species of tourmaline based on chemical composition: buergerite, chromdravite, dravite, elbaite, feruvite, foitite, liddicoatite, olenite, povondraite, schorl and uvite. The exact species of tourmaline is determined by which of a number of possible elements are present. Tourmaline occurs as lustrous, elongate crystals which commonly have a rounded triangular cross section and narrow grooves running parallel to their long direction. The crystals range in size from microscopic to over a foot long.
Tourmaline is Maine's state mineral and the birthstone for the month of October. Elbaite was named after the isle of Elba in Italy.
Uses:
- Tourmaline is used as a gemstone, occuring in all colors.
- Tourmaline also has many scientific and technological uses because an electrical charge can be induced in some tourmaline crystals by applying pressure to the crystal in the direction of the vertical crystal axis. This effect is known as piezoelectricity, and has many uses in pressure-measuring equipment and other scientific applications. Some tourmalines also show pyro-electricity, which occurs when the crystal is heated, yielding a positive charge at one end of the crystal and a negative charge at the other.
 Moss Agate — SiO2
Moss Agate — SiO2
Agate is a quartz gem, classified as microcrystalline quartz. Agate comes from silica, which comes from volcanoes. The ash, water, rain, manganese, iron, and other minerals of the earth form together to create agate under pressure and heat. Silica is always predominant in agate, usually with aluminum and oxide of iron present as well. An agate's banding forms as silica from solution is slowly deposited into cavities and veins in older rock. Important sources of agate are Brazil, Uruguay, and the United States (Oregon, Washington, and around Lake Superior).
Agates range from transparent to opaque in a variety of beautiful colors. Agate presents various tints in the same specimen. The stones can be artificially stained to produce combinations of color more vivid than those found in the natural state. Physical properties of agate are in general those of quartz. Agate has irregular, sometimes circular bands of color and often replaces fossil wood. Many fossils are agatized material where the original organic substance has been replaced by agate while retaining the original structure.
Agates are identical in chemical structure to jasper, flint, chert, bloodstone, and tiger-eye, and are often found in association with opal.
The moss agate or mocha stone contains visible impurities in the form of dendritic shapes that resemble moss. Agate is a hard gemstone, found in all colors of the rainbow, although green and blue are the more rare shades.
Uses:
- The colorful, banded rocks are used as a semiprecious gemstone and in the manufacture of grinding equipment (mortar and pestles).
- Agate was used for thousands of years, first as a decoration and then as ornamental jewelry.
- Agate is the gemstone for the 12th anniversary of marriage.
- Moss agate is the gemstone for the 14th anniversary of marriage.
- Agate is used as a birthstone for both May and June.
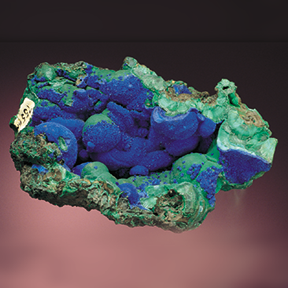 Malachite
— CH2Cu2O5)
Malachite
— CH2Cu2O5)
Malachite is a famous and very popular semi-precious stone. It is named for the Greek word for "mallow," a green herb. Its banded light and dark green designs are one-of-a-kind, and give it a unique ornamental quality unlike that of any other stone. The light and dark green bands are so distinctive that malachite may be one of the minerals most easily recognized by the general public.
A popular design of ceramic ware, which imitates this banding, is named after the mineral malachite. It forms the banding from subtle changes in the oxidation states of the surrounding pore waters, but the exact mechanism is still not well understood.
Alternative names:
- Bremen green
- olympian green
- iris green
Uses:
- As mineral specimens, an important ore of copper
- an ornamental stone
- a pigment
- for jewelry
 Smoky Quartz — SiO2
Smoky Quartz — SiO2
Nearly 2000 years ago Pliny wrote that quartz crystals formed from ice exposed to intense cold for long periods of time in dark clefts and caverns in the mountains. This general belief was popular in diverse cultures until the 18th century, when modern geology began to develop in Europe.
The very dark brown to black smoky quartz is sometimes called morion. Faceted brown smoky quartz has been called smoky topaz, and also is known as cairngorm, named after the Cairngorm Mountains in the Scottish highlands.
>The name quartz comes from the Saxon word "querklufterz," which meant cross vein ore.
<Smoky quartz is a macrocrystalline variety of the mineral quartz (SiO2). Quartz is the most abundant single mineral on earth. It makes up about 12% of the earth's crust, occurring in a wide variety of igneous, metamorphic, and sedimentary rocks.
Quartz varieties are commonly separated into two groups based on the size of the individual grains or crystals: macrocrystalline quartz, in which individual crystals are distinguishable with the naked eye, and cryptocrystalline quartz, in which the individual crystals are too small to be easily distinguishable under the light microscope.
Uses:
- Smoky quartz has been used as a gemstone and in ornamental and religious objects for thousands of years.
 Rhodochrosite
— MnCO3
Rhodochrosite
— MnCO3
Rhodochrosite belongs to the calcite group, a group of related carbonates that are isomorphous with one another. They are similar in many physical properties and may partially or fully replace one another, forming a partial solid solution series. All members of the calcite group crystallize in the trigonal subdivision of the hexagonal system (as rhombohedrons and scalenohedrons), have perfect rhombohedral cleavage, and exhibit a strong double refraction in transparent rhombohedrons.
When rhodochrosite is exposed to the atmosphere, it develops a thin film of manganese oxide on its surface. This may slightly darken the color of a specimen. Rhodochrosite commonly alters into black manganese oxides (such as pyrolusite, manganite, and psilomelane), and black manganese oxide stains are usually associated with rhodochrosite.
Rhodochrosite most commonly forms in hydrothermal veins associated with silver, copper, and lead sulfides, but rarely occurs in pegmatites.
An interesting occurrence of this mineral is in Argentina, where rhodochrosite forms stalagmites and stalactites in the ancient Inca silver mines.The Colorado and South African rhodochrosite mines have produced specimens that many consider the most beautiful of all minerals. They occur in blood-red, transparent to translucent, perfectly shaped rhombohedral and scalenohedral crystals.
Uses:
- Rhodochrosite is faceted into cut stones, but only for collectors. However, the banded stalactitic material from Catamarca, Argentina is carved into ornaments and polished into cabochons and beads for jewelry. Fine, blood-red rhodochrosite specimens are highly desired by collectors, and command magnificent prices.
- Rhodochrosite is also used as an ore of manganese.
Synonym:
- Manganese spar
 Amethyst — (SiO2)
Amethyst — (SiO2)
Amethyst is a well-known gem. It is a purple variety of quartz, which includes many other gemstones, such as citrine, smoky quartz, and rock crystal. The color of amethyst specimens from certain localities slowly fade upon prolonged exposure to light. Much citrine is artificially formed by heat-treating amethyst.
Uses:
- Most amethyst is faceted into jewelry cuts, and some are cut as cabochons. Large, massive chunks of amethyst banded with quartz are sometimes carved into ornaments.
- Amethyst also is popular among mineral collectors. Small geode sections and tumbled stones are sold to amateur collectors, while more experienced collectors go for the rare prismatic crystals and giant geode sections.
 Chalcopyrite — CuFeS2
Chalcopyrite — CuFeS2
From the Greek words chalkos, "copper" and pyrite, "strike fire." Chalcopyrite is the most abundant copper-bearing mineral and is widespread. It is a primary mineral in hydrothermal veins, desseminations, and massive replacements, and the principal copper mineral of porphyry-copper deposits. Its color is brass yellow, often with an iridescent tarnish.
Uses:
- It is the principal ore of copper.
- Chalcopyriteis a popular mineral among collectors. Fine specimens, such as those from Arizona, Missouri, and Mexico may have well-formed tetrahedrons measuring as much as four or five inches across.
Synonym:
- Peacock Copper
 Fluorite — CaF2
Fluorite — CaF2
Deep purple, amethyst, sky blue, sea green, sunny yellow, and crystal They're "clear" — the
mineral fluorite  comes in all colors. Many types of fluorite even glow
under ultraviolet light. "fluorescent."
comes in all colors. Many types of fluorite even glow
under ultraviolet light. "fluorescent."
Pure fluorite, made of the elements calcium (Ca) and fluorine (F), is colorless. The various colors result from tiny amounts of other elements substituting for the calcium in the crystalline structure.
Fluorite is found as a common gangue mineral in hydrothermal veins, especially those containing lead and zinc minerals. It is also found in some greisens, granites, and high-temperature veins, and as a component of some marbles and other metamorphic rocks.
Fluorite is the state mineral of Illinois.
Uses:
- As a flux (hence the name) in iron smelting
- as a rare gemstone
- as a source of fluorine
- as special optical lenses
- as a popular mineral specimen
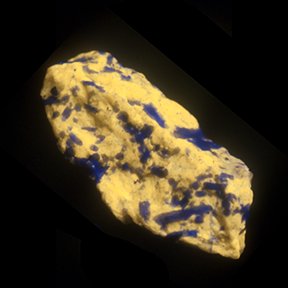
Halite —NaCl
Halite, better known as rock salt, can easily be distinguished by its taste. There is a right way to taste a specimen of halite (or an unknown mineral that is similar to halite) and a wrong way. The right way is to first lick your index finger, rub it against the specimen and then taste the finger. This limits the amount of the mineral that actually gets in your mouth, an important consideration when you consider that there are poisonous minerals that resemble halite.
Halite is part of the halide group of minerals. Halide minerals contain one of the halogen elements (chlorine, fluorine, bromine, and iodine) as a building block. Most halides are soft and fragile, and some are soluble in water. Many crystallize in the isometric system.
Haliteis found in many current evaporative deposits such as near Salt Lake City, Utah and Searles Lake, California in the U.S., where it crystallizes out of evaporating brine lakes. It is also found in ancient bedrock all over the world where large extinct salt lakes and seas have evaporated millions of years ago, leaving thick deposits of salt behind.
The cities of Cleveland and Detroit rest above huge halite deposits that are mined for road salt. In some underground salt deposits in Texas and Louisiana, the salt is pushed upwards by an underground force through soft ground and forms arched structures known as "salt domes."
Uses:
- As food seasoning
- for road safety to melt snow and ice
- as salt licks for cattle (these provide the cattle with salt, which is essential to their health), and for medicinal purposes
- In purification, potassium, magnesime salts, bromine, and iodine are obtained as by-products.

